Trastuzumab Deruxtecan in Metastatic Breast Cancer with Variable HER2 Expression: the Phase 2 DAISY Trial
Fernanda Mosele, Elise Deluche, Amelie Lusque, Loïc Le Bescond, Thomas Filleron, Yoann Pradat, Agnes Ducoulombier, Barbara Pistilli, Thomas Bachelot, Frederic Viret, Christelle Levy, Nicolas Signolle, Alexia Alfaro, Diep TN Tran, Ingrid Judith Garberis, Hugues Talbot, Stergios Christodoulidis, Maria Vakalopoulou, Nathalie Droin, Aurelie Stourm, Maki Kobayashi, Tomoya Kakegawa, Ludovic Lacroix, Patrick Saulnier, Bastien Job, Marc Deloger, Marta Jimenez, Celine Mahier, Vianney Baris, Pierre Laplante, Patricia Kannouche, Virginie Marty, Magali Lacroix-Triki, Veronique Diéras, Fabrice André
Nature Medicine, 2023
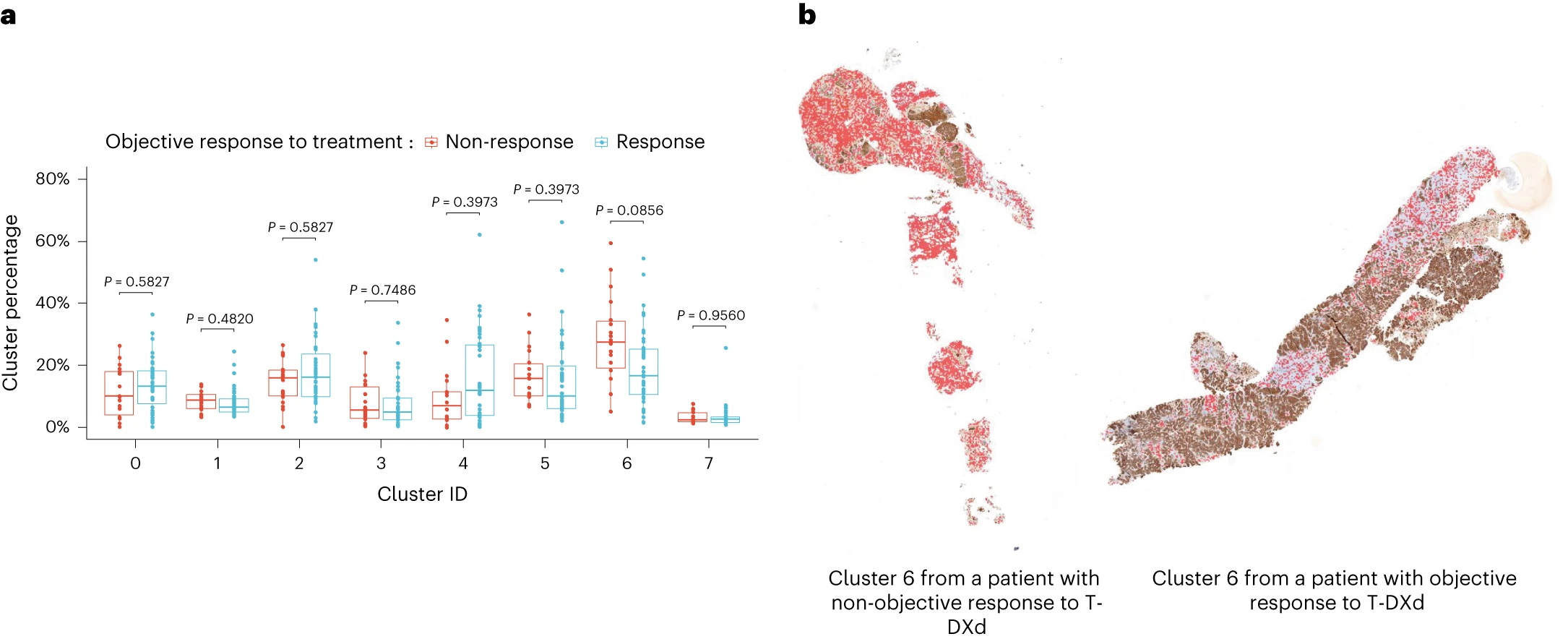
Abstract: The mechanisms of action of and resistance to trastuzumab deruxtecan (T-DXd), an anti-HER2–drug conjugate for breast cancer treatment, remain unclear. The phase 2 DAISY trial evaluated the efficacy of T-DXd in patients with HER2-overexpressing (n = 72, cohort 1), HER2-low (n = 74, cohort 2) and HER2 non-expressing (n = 40, cohort 3) metastatic breast cancer. In the full analysis set population (n = 177), the confirmed objective response rate (primary endpoint) was 70.6% (95% confidence interval (CI) 58.3–81) in cohort 1, 37.5% (95% CI 26.4–49.7) in cohort 2 and 29.7% (95% CI 15.9–47) in cohort 3. The primary endpoint was met in cohorts 1 and 2. Secondary endpoints included safety. No new safety signals were observed. During treatment, HER2-expressing tumors (n = 4) presented strong T-DXd staining. Conversely, HER2 immunohistochemistry 0 samples (n = 3) presented no or very few T-DXd staining (Pearson correlation coefficient r = 0.75, P = 0.053). Among patients with HER2 immunohistochemistry 0 metastatic breast cancer, 5 of 14 (35.7%, 95% CI 12.8–64.9) with ERBB2 expression below the median presented a confirmed objective response as compared to 3 of 10 (30%, 95% CI 6.7–65.2) with ERBB2 expression above the median. Although HER2 expression is a determinant of T-DXd efficacy, our study suggests that additional mechanisms may also be involved. (ClinicalTrials.gov identifier NCT04132960.)
@article{mosele2023trastuzumab,
title={Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial},
author={Mosele, Fernanda and Deluche, Elise and Lusque, Amelie and Le Bescond, Lo{\"\i}c and Filleron, Thomas and Pradat, Yoann and Ducoulombier, Agnes and Pistilli, Barbara and Bachelot, Thomas and Viret, Frederic and others},
journal={Nature Medicine},
pages={1--11},
year={2023},
publisher={Nature Publishing Group US New York}
}